Products provided by OrphanPacific
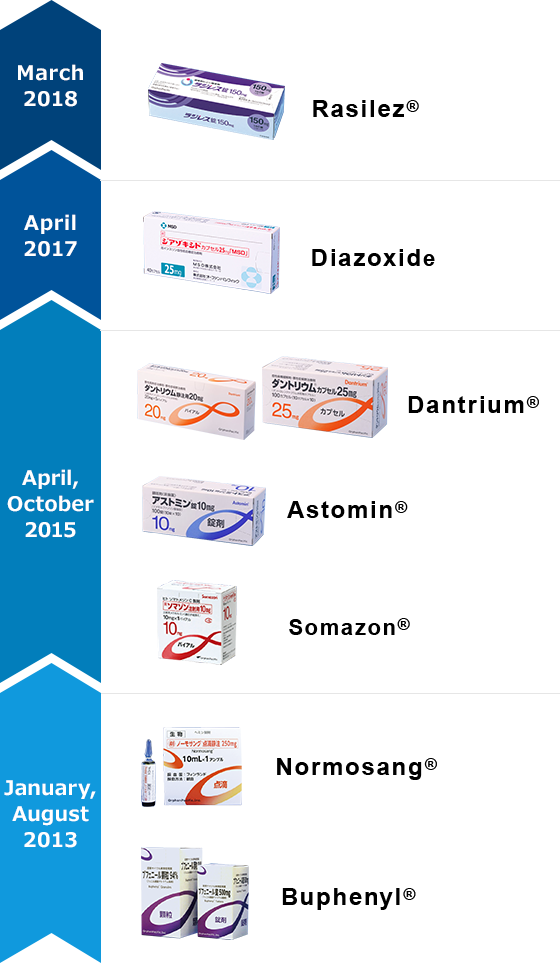
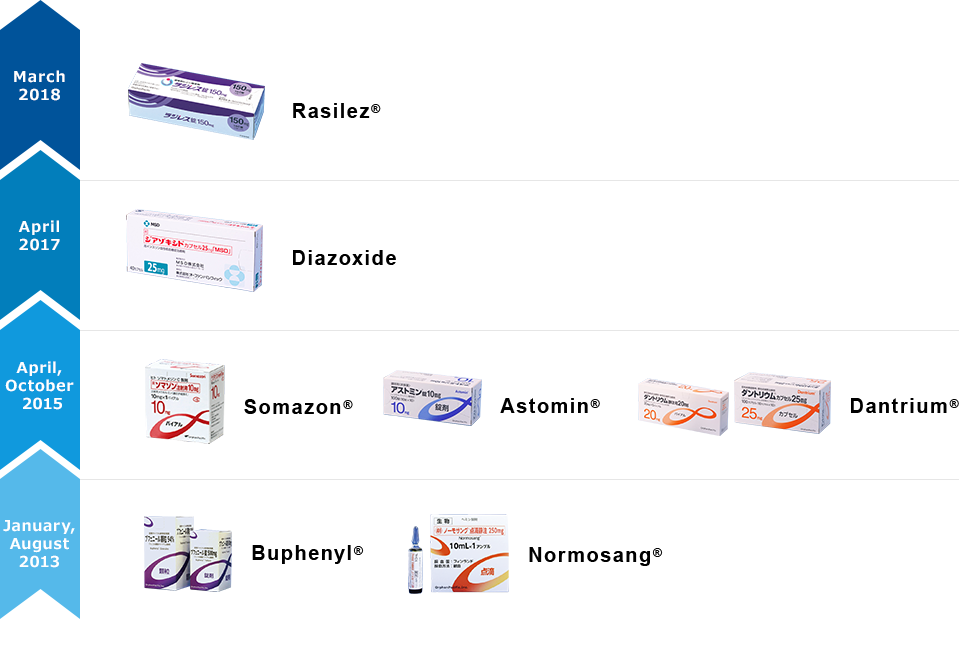
| Trade Name (Generic Name) |
Formulation | Rare Disease6) |
Classification of drugs by efficacy or indication |
Number of patient | First commercial sales |
|---|---|---|---|---|---|
| Radilez® (Aliskiren Fumarate) |
150mg Tablet | Hypertension | - | March 1, 2018 | |
| Diazoxide(Diazoxide) | 25mg Capsule | ○ | Hyperinsulinemic Hypoglycemia | (Persistent) 1/35400 Born/Year5) |
April 1, 2017 |
| Dantrium® (Dantrolene Sodium Hydrate) |
20mg Intravenous Injection |
○ | Malignant Hyperthermia | 4~5/Year4) | October 1, 2015 |
| ● | Neuroleptic Malignant Syndrome | 100~300/Year3) | |||
| 25mg Capsule | |||||
| Spastic paralysis, Syndrome of Progressive Muscle Spasm |
- | ||||
| Astomin® (Dimemorfan Phosphate) |
10mg Tablet | Acute and Chronic Cough | - | ||
| 10% Powder | |||||
| 0.25% Syrup | |||||
| Somazon®(Mecasermin) | 10mg Injection | ● | Insulin Receptor Abnormalities, Growth Hormone Resistant Dwarfism |
283) | April 1, 2015 |
| Normosang®(Hemin) | 250mg Infusion | ● | Acute Porphyria | 362) | August 23, 2013 |
| Buphenyl® (Sodium Phenylbutyrate) |
500mg Tablet | ● | Urea Cycle Disorders | 2861) | January 17, 2013 |
| 94% Granule | ● |
1)Kato.et al., The status of nationwide registration of research projects for Specific Pediatric Chronic Diseases 2011.
2)Kawada et al., National epidemiological survey on hereditary porphyria (primary investigation) Result report
3)Edited by Orphan Drag Study Group, Handbook of Rare Diseases, Pharmaceutical Handbook 2009, pp. 39-40, Jiho, 2009
4)Mukaida et al., Journal of Japan Society of Clinical Anesthesia 32(5), 682-690, 2012
5)Kawakita et al., Journal of Japan Pediatric Society 115(3), 563-569, 2011
6)● indicates a disease designated as orphan drug, ○ indicates a disease with less than 50,000 patients
Contact us
Monday to Friday 9:00 a.m. to 5:30 p.m. on weekdays (Japan time).




